Vani Hari, AKA the FoodBabe, shared on her Facebook page today a post about 'toxic' glucose solutions used in OGTTs for pregnant women. You can find her post here. The article focuses on the brominated vegetable oil (BVO) content of this drink, after expressing delight that Coke/Pepsi are removing BVO from their products, which I'm sure she'll now consume copious amounts of..
Before we get into the toxicity data on BVO, why is BVO added to drinks? Well, it's not to a government-conspiracy to poison consumers. Citrus oils are added to beverages, usually soft drinks, as a clouding/flavor agent. However, these oils are less dense than the water-sugar mixture, so small amounts of brominated vegetable oil are dissolved in the citrus oil to increase their density and keep them in suspension. Alternative weighting agents do exist in the forms of damar gums, ester gums, and sucrose diacetate hexaisobutyrate (Fennema's Food Chemistry,4th Edition, 2008).
Regarding the toxicity of BVO, you can find its history chronicled in this ScientificAmerican Article here. The FDA approved levels are 15ppm in beverages, after reviewing toxicology studies in animal models that showed no effect at 1500ppm, leaving a 100-fold window of safety (1). You can find case studies (2,3) of individuals presenting with bromism after consistently consuming several liters of BVO-containing sodas per day. There is also data from human studies that have looked at organo-bromoine levels in tissues(adipose, liver, spleen, brain, and kidneys -obtained post-mortem) of individuals of varying ages from the UK, Germany and Holland (n=374) (4). Individuals from the UK had the highest levels, at a time when the UK allowed 80ppm in soft drinks. This data did not show any health outcomes related to the BVO content of tissues. People, and groups like CSPI, have caused a big stink about BVO in recent years because new data on the topic hasn't been generated in decades, apart from these case studies finding that very excessive consistent consumption of drinks containing BVO have led to individual medical cases. Snopes covered this topic here, quoting CSPI's executive director as saying "Is it harmful at the amounts consumed? Probably not," Jacobson said. "But it would be nice if the FDA did a thorough review of the literature and finalized an approval or a ban."
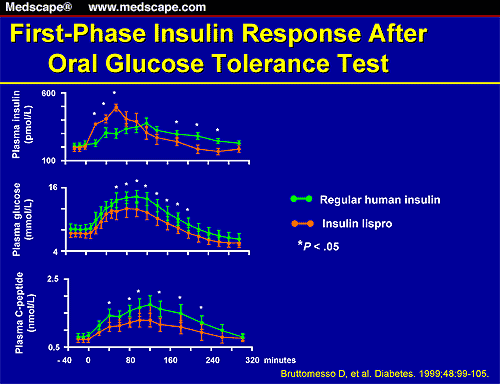
In short, we've got very rare cases of people consuming excessive amounts of soda and fruit drinks for extended periods of time who react to BVO. We've got animal toxicology data that has led the FDA to conclude 15ppm is a safe upper threshold. And we've got some people who would like more data generated on BVO. Oh, and as always, we've got Dr. Joe Mercola inspiring fear with as many outlandish claims as possible about bromine (see here).
Would I like more data on BVO? Sure. But in the case of BVO, I would simply argue that you've got bigger issues, due to a nutritionally unbalanced diet, if you're consuming enough soda or fruit drinks that you'd be putting yourself at risk for bromism. What I wouldn't argue is that a simple one time test with a solution that contains BVO is harmful or something that should be actively avoided.
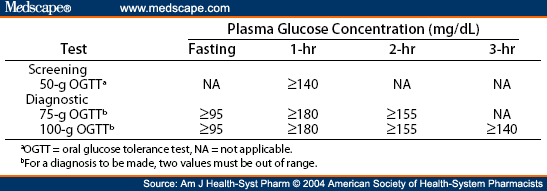
Ms. Hari and several of the commenters have made suggestions for alternatives to OGTTs. I think one should note that it's important in medicine to have things standardized. Diagnosing an individual with gestational diabetes is done by measuring his/her blood glucose response following a bolus of glucose - using something like a banana hasn't been standardized, and further, all bananas don't contain the same amount of sugar (also, absorption will be modified by the amount of fiber/starch content, and blood sugar response will be different due to the glucose:fructose content). We know that someone who is insulin sensitive will rapidly absorb pure glucose, have a predictable rise in that blood sugar, and a clear fall, and there will be a clear deviation from this in someone who is insulin resistant. The following shows the criteria for diagnosing gestational diabetes.
Natural Flavors: You can read all about what those are here. We don't really know too much about which one are used here without contacting the company. I cannot find any data that suggests cause for concern from a one-time ingestion.
Modified Food Starch: This could represent any number of modifications to starch granules, probably added to increase the viscosity of the liquid for mouth-feel. You can read more about the types here. We don't know much about the one used here. I cannot find any data that suggests cause for concern from a one-time ingestion.
Sodium Hexametaphosphate (6): The amounts in foods are not considered toxic. These salts are hydrolyzed to sodium and phosphoric acids in the gut. The most recent toxicology report on Sodium Hexametaphosphate shows severe kidney issues with consistent large intakes in mice, due to mineral imbalances (7). None of these concerns apply to one time consumption of an oral glucose solution.
Yellow #6: Yellow 6 has an ADI of 3.75 mg/kg bw/day and intakes are estimated at an average 14mg/day (13). Acute oral toxicity (LD50 )of Yellow #6 occurs at 5,000mg/kg (8). Ms. Hari notes that these come from Petroleum, which just illustrates her lack of chemistry knowledge. Petroleum is a mix of hydrocarbons that are utilized for making any number of products - supposedly you can even synthesize vitamins from it (9). Controversy surrounding Yellow #6 relates to its potential role in hyperactivity of children and benzidine content - see here. None of the controversies apply to one time consumption of an oral glucose solution.
BHA (10): Butylated Hydroxyanisole is a potent antioxidant added to preserve the shelf-life of foods. The LD50 is 2g/kg for mice, rabbits and rats. It does not have teratogenic effects in mammals. Chronic ingestion of high doses causes cancer in rats, mice and hamsters - this cancer is found in the forestomach, an organ that humans don't have. Since we love Europe and its standards, the European Food Safety Authority sets the acceptable daily intake for BHA at 1mg/kg/bw/day, an intake that food is not likely to achieve, and is 100x lower than doses that showed no observable affects in animal models (11). None of these concerns apply to a one time consumption of an oral glucose solution.
Sodium Benzoate: Acceptable daily intake is set by the WHO at 5mg/kg (12) and the FDA sets sodium benzoate levels at .1% of a product by weight. It is found naturally in apples and cranberries in very low amounts. The concern over sodium benzoate tends to surround the fact that it can form Benzene in the presence of vitamin C - you can read more about this here, and this product doesn't appear to contain vitamin C. None of these concerns apply to a one time consumption of an oral glucose solution.
Again, none of these other ingredients pose any known risk from a one time consumption. Referring to this glucose syrup as 'toxic', which Ms Hari does, is entirely unfounded and unscientific. It is my hope that no one who reads her article refuses a glucose tolerance test, as gestational diabetes has severe detrimental outcomes for both mom and the baby - see the CDC pamphlet here. And in the future, if you'd like medical advice, see a team of physicians and registered dietitians. And if you want to know about toxicology, find a toxicologist.
If you're someone who is concerned about these ingredients in food, you should be concerned about Ms. Hari's statements as well. The concerns surrounding these ingredients are due to their chronic effects, not a one time she solution. Her misrepresentation of the science surrounding these ingredients only detracts from legitimate conversations about chronic toxicity. The last thing we need is a more polarized debate surrounding these ingredients, and these sort of inflammatory posts only ensure that that is all we will have.
1. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?FR=180.30
2.http://www.nejm.org/doi/full/10.1056/NEJM200305083481921
3. http://informahealthcare.com/doi/abs/10.3109/15563659709001219
4. http://journals.cambridge.org/action/displayAbstract?fromPage=online&aid=832064
5. http://www.ncbi.nlm.nih.gov/pubmed/10561636
6. http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=24968#x332
7. http://www.ncbi.nlm.nih.gov/pubmed/11766135
8. http://www.durhamtech.edu/faculty/safety/MSDS%20Files/MSDS%20-%20Chemistry/FD&C%20Yellow%20%236%202783-94-0%20MSDS.pdf
9. http://www.foodinsight.org/blogs/information-key-overcoming-food-fears
10. http://www.accessdata.fda.gov/scripts/fcn/fcnDetailNavigation.cfm?rpt=scogsListing&id=40
11. http://www.efsa.europa.eu/en/efsajournal/pub/2759.htm
12. http://www.ncbi.nlm.nih.gov/pubmed/11766131
13. http://www.gpo.gov/fdsys/pkg/FR-1995-07-21/html/95-17831.htm
Before we get into the toxicity data on BVO, why is BVO added to drinks? Well, it's not to a government-conspiracy to poison consumers. Citrus oils are added to beverages, usually soft drinks, as a clouding/flavor agent. However, these oils are less dense than the water-sugar mixture, so small amounts of brominated vegetable oil are dissolved in the citrus oil to increase their density and keep them in suspension. Alternative weighting agents do exist in the forms of damar gums, ester gums, and sucrose diacetate hexaisobutyrate (Fennema's Food Chemistry,4th Edition, 2008).
Regarding the toxicity of BVO, you can find its history chronicled in this ScientificAmerican Article here. The FDA approved levels are 15ppm in beverages, after reviewing toxicology studies in animal models that showed no effect at 1500ppm, leaving a 100-fold window of safety (1). You can find case studies (2,3) of individuals presenting with bromism after consistently consuming several liters of BVO-containing sodas per day. There is also data from human studies that have looked at organo-bromoine levels in tissues(adipose, liver, spleen, brain, and kidneys -obtained post-mortem) of individuals of varying ages from the UK, Germany and Holland (n=374) (4). Individuals from the UK had the highest levels, at a time when the UK allowed 80ppm in soft drinks. This data did not show any health outcomes related to the BVO content of tissues. People, and groups like CSPI, have caused a big stink about BVO in recent years because new data on the topic hasn't been generated in decades, apart from these case studies finding that very excessive consistent consumption of drinks containing BVO have led to individual medical cases. Snopes covered this topic here, quoting CSPI's executive director as saying "Is it harmful at the amounts consumed? Probably not," Jacobson said. "But it would be nice if the FDA did a thorough review of the literature and finalized an approval or a ban."

In short, we've got very rare cases of people consuming excessive amounts of soda and fruit drinks for extended periods of time who react to BVO. We've got animal toxicology data that has led the FDA to conclude 15ppm is a safe upper threshold. And we've got some people who would like more data generated on BVO. Oh, and as always, we've got Dr. Joe Mercola inspiring fear with as many outlandish claims as possible about bromine (see here).
Would I like more data on BVO? Sure. But in the case of BVO, I would simply argue that you've got bigger issues, due to a nutritionally unbalanced diet, if you're consuming enough soda or fruit drinks that you'd be putting yourself at risk for bromism. What I wouldn't argue is that a simple one time test with a solution that contains BVO is harmful or something that should be actively avoided.
Ms. Hari and several of the commenters have made suggestions for alternatives to OGTTs. I think one should note that it's important in medicine to have things standardized. Diagnosing an individual with gestational diabetes is done by measuring his/her blood glucose response following a bolus of glucose - using something like a banana hasn't been standardized, and further, all bananas don't contain the same amount of sugar (also, absorption will be modified by the amount of fiber/starch content, and blood sugar response will be different due to the glucose:fructose content). We know that someone who is insulin sensitive will rapidly absorb pure glucose, have a predictable rise in that blood sugar, and a clear fall, and there will be a clear deviation from this in someone who is insulin resistant. The following shows the criteria for diagnosing gestational diabetes.
While things like Jelly Beans have been demonstrated (5) as a substitute to a sugar syrup, it's not likely that your doctors office will provide that. Having a standardized solution on hand that has a long shelf-life and doesn't require the patient to bring in their own alternative is important to have for a doctor's office and that's why these products exist. If you want to go out of your way to bring in your own GMO, all natural, "nasties"-free Jelly Beans, that's fine. But instilling fear in individuals over a one time consumption of a drink containing BVO is ridiculous.
As far as the other ingredients go, Ms. Hari highlights dextrose...which is the sugar (its another name for D-Glucose, the D refers to the stereochemistry of the glucose molecule) and is found naturally in honey (she notes that it probably comes from corn, which is probably alluding to the fact that its GMO, which doesn't matter because this doesn't contain protein - it's chemically indistinguishable from other sources of dextrose). She also highlights that it contains Natural Flavorings, Modified Food Starch, Yellow #6, Sodium Hexametaphosphate (a sequestrant and texturizer used in foods that adds a small amount of sodium), BHA, and Sodium Benzoate.
Modified Food Starch: This could represent any number of modifications to starch granules, probably added to increase the viscosity of the liquid for mouth-feel. You can read more about the types here. We don't know much about the one used here. I cannot find any data that suggests cause for concern from a one-time ingestion.
Sodium Hexametaphosphate (6): The amounts in foods are not considered toxic. These salts are hydrolyzed to sodium and phosphoric acids in the gut. The most recent toxicology report on Sodium Hexametaphosphate shows severe kidney issues with consistent large intakes in mice, due to mineral imbalances (7). None of these concerns apply to one time consumption of an oral glucose solution.
Yellow #6: Yellow 6 has an ADI of 3.75 mg/kg bw/day and intakes are estimated at an average 14mg/day (13). Acute oral toxicity (LD50 )of Yellow #6 occurs at 5,000mg/kg (8). Ms. Hari notes that these come from Petroleum, which just illustrates her lack of chemistry knowledge. Petroleum is a mix of hydrocarbons that are utilized for making any number of products - supposedly you can even synthesize vitamins from it (9). Controversy surrounding Yellow #6 relates to its potential role in hyperactivity of children and benzidine content - see here. None of the controversies apply to one time consumption of an oral glucose solution.
BHA (10): Butylated Hydroxyanisole is a potent antioxidant added to preserve the shelf-life of foods. The LD50 is 2g/kg for mice, rabbits and rats. It does not have teratogenic effects in mammals. Chronic ingestion of high doses causes cancer in rats, mice and hamsters - this cancer is found in the forestomach, an organ that humans don't have. Since we love Europe and its standards, the European Food Safety Authority sets the acceptable daily intake for BHA at 1mg/kg/bw/day, an intake that food is not likely to achieve, and is 100x lower than doses that showed no observable affects in animal models (11). None of these concerns apply to a one time consumption of an oral glucose solution.
Sodium Benzoate: Acceptable daily intake is set by the WHO at 5mg/kg (12) and the FDA sets sodium benzoate levels at .1% of a product by weight. It is found naturally in apples and cranberries in very low amounts. The concern over sodium benzoate tends to surround the fact that it can form Benzene in the presence of vitamin C - you can read more about this here, and this product doesn't appear to contain vitamin C. None of these concerns apply to a one time consumption of an oral glucose solution.
Again, none of these other ingredients pose any known risk from a one time consumption. Referring to this glucose syrup as 'toxic', which Ms Hari does, is entirely unfounded and unscientific. It is my hope that no one who reads her article refuses a glucose tolerance test, as gestational diabetes has severe detrimental outcomes for both mom and the baby - see the CDC pamphlet here. And in the future, if you'd like medical advice, see a team of physicians and registered dietitians. And if you want to know about toxicology, find a toxicologist.
If you're someone who is concerned about these ingredients in food, you should be concerned about Ms. Hari's statements as well. The concerns surrounding these ingredients are due to their chronic effects, not a one time she solution. Her misrepresentation of the science surrounding these ingredients only detracts from legitimate conversations about chronic toxicity. The last thing we need is a more polarized debate surrounding these ingredients, and these sort of inflammatory posts only ensure that that is all we will have.
1. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?FR=180.30
2.http://www.nejm.org/doi/full/10.1056/NEJM200305083481921
3. http://informahealthcare.com/doi/abs/10.3109/15563659709001219
4. http://journals.cambridge.org/action/displayAbstract?fromPage=online&aid=832064
5. http://www.ncbi.nlm.nih.gov/pubmed/10561636
6. http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=24968#x332
7. http://www.ncbi.nlm.nih.gov/pubmed/11766135
8. http://www.durhamtech.edu/faculty/safety/MSDS%20Files/MSDS%20-%20Chemistry/FD&C%20Yellow%20%236%202783-94-0%20MSDS.pdf
9. http://www.foodinsight.org/blogs/information-key-overcoming-food-fears
10. http://www.accessdata.fda.gov/scripts/fcn/fcnDetailNavigation.cfm?rpt=scogsListing&id=40
11. http://www.efsa.europa.eu/en/efsajournal/pub/2759.htm
12. http://www.ncbi.nlm.nih.gov/pubmed/11766131
13. http://www.gpo.gov/fdsys/pkg/FR-1995-07-21/html/95-17831.htm

Comments
Post a Comment